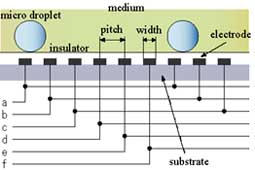
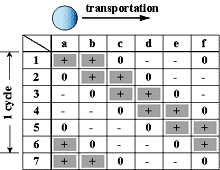
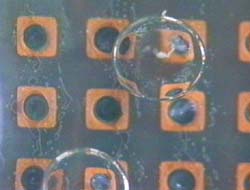
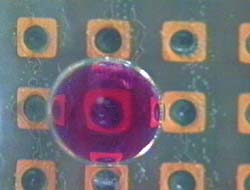
On the other hand, since in a new method developed in this research, only a minimum volume of chemical solutions to react was put into micro droplets, this method has advantages in sample/reagent volume, reaction time, and cost. And, since these micro droplets were manipulated in chemically stable liquid, some problems, such as evaporation and contaminations, which were not negligible in case in the open air, can be avoided. Further, since micro droplets were manipulated independently, and two dimensionally by electrostatic force on the devices, on which electrodes were patterned, micro fluidic devices, such as micropumps, and microvalves, were not necessary and a structure of the device can be simple. Since using this method many chemical reactions can be caused on one chip simultaneously, the "Lab on a chip" device for combinatorial chemistry can be achieved.
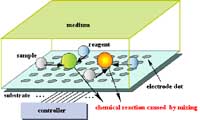
Fig. 1 "Lab on a Chip" device for combinatorial chemistry